Kuvaus
Product Type:
Stimulator / wearable cardiac muscle stimulator
Areas of Application:
- permanent control by the wearable defibrillator for emergency treatment of cardiac arrest and ventricular fibrillation
Indications:
(according to manufacturer's instructions):
- high risk of PHT/SCD immediately after explantation of an implanted defibrillator
- high risk of PHT/SCD in whom immediate implantation of an ICD is indicated but not possible for medical reasons
- high risk of PHT/SCD with right ventricular or right atrial thrombus or tumor (myxoma)
- planned heart transplantation in patients
Description:
The system continuously monitors heart activity and warns of any abnormalities by voice command. If the affected person does not react, resuscitation begins automatically - even if they are unconscious.
If the process is stopped manually, unnecessary treatment shocks are prevented in the event of incorrect arrhythmia detection. It takes less than a minute from detection to shock delivery. If the arrhythmia persists, up to five further shocks can follow.
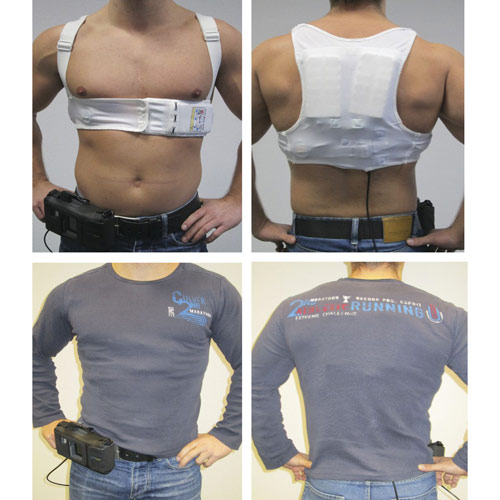
The system is worn as a vest with a belt to which a monitor is attached for monitoring. The data is automatically transmitted to the LifeVest network and can be viewed online by the treatment team.
Features / components:
- Technology: Automated External Defibrillator (AED)
- Defibrillator: fully automatic
- Operation: no intervention by other people necessary
- Electrodes: but automatically gel out before a shock
- LifeVest-Network: wireless data transmission and control
Price (without guarantee):
The price is available on request from the manufacturer / distributor.